Phases or Matter
Matter
Matter is anything that has mass and takes up space. It is made up of tiny particles called atoms and molecules, which combine in different ways to form solids, liquids, gases, and plasma. Matter can undergo physical and chemical changes, but its total amount remains constant due to the law of conservation of mass. Anything in the universe that has mass is made of matter.
Phases
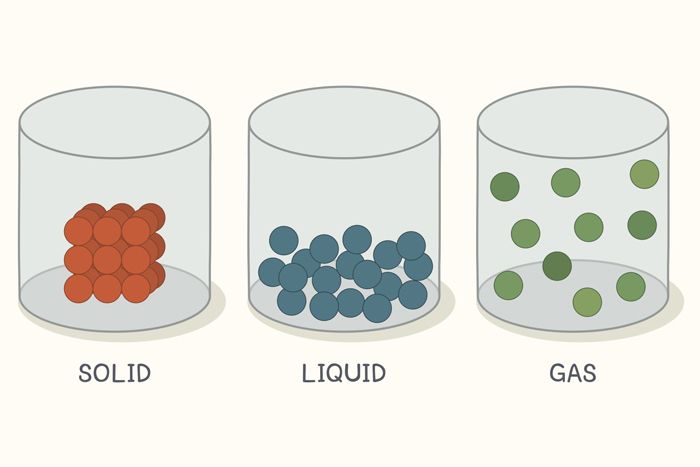
Matter exists in four main phases: solid, liquid, gas, and plasma. In the solid phase, particles are tightly packed and vibrate in place, giving solids a fixed shape and volume. In the liquid phase, particles are more loosely arranged, allowing them to flow and take the shape of their container while maintaining a constant volume. In the gas phase, particles move freely and spread out to fill any container, having neither a fixed shape nor volume. The plasma phase, found in stars and lightning, consists of highly energetic, ionized particles that conduct electricity. Transitions between these phases occur due to changes in temperature and pressure.
Phase Changes
Phase changes of matter occur when a substance transitions between solid, liquid, gas, and plasma due to temperature or pressure changes. The main phase changes include melting (solid to liquid), freezing (liquid to solid), vaporization (liquid to gas), condensation (gas to liquid), sublimation (solid to gas), and deposition (gas to solid). These changes involve energy absorption or release, influencing particle movement and arrangement.